- Boxing
- Athletics
- Basketball
- Bodybuilding
- Cricket
- Golf
- Handball
- Hockey
- Martial Arts
- Tennis
- Volleyball
- Other Sports

Africa News of Thursday, 24 April 2025
Source: www.ghanawebbers.com
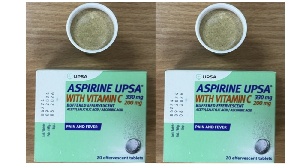
Rwanda Recalls French-Manufactured Aspirin Over Quality Concerns
The Rwanda Food and Drugs Authority has announced a recall.
This affects batch No. B6224 of Aspirine Vitamin C 330mg/200mg effervescent tablets.
The product is made by Union de pharmacologie scientifique appliquée (UPSA), a French company.
The recall is due to quality concerns.
On April 23, Rwanda FDA issued a statement about the issue.
They received complaints regarding the affected batch, produced in September 2024.
This batch is set to expire in August 2026.
Investigations revealed that the tablets changed color from white to brown.
This change compromises the product's quality standards.
Rwanda FDA has ordered an immediate halt to distribution and dispensing of this batch.
Importers, distributors, and health facilities must return the recalled products.
They need to report back to Rwanda FDA within 10 working days.
The report should include quantities imported, distributed, returned, and remaining stock.
The public is advised not to use any products from this batch.
For more information, stakeholders can contact Rwanda FDA directly.
Entertainment